Embark on a journey of scientific exploration with our intensive and extensive properties worksheet, an indispensable tool for understanding the fundamental characteristics of matter. This worksheet delves into the intricacies of these properties, empowering you with a deeper comprehension of the physical world.
As we delve into the concepts of intensive and extensive properties, we will unravel their unique characteristics and explore their contrasting behaviors. Prepare to be captivated as we uncover the applications of these properties in diverse fields, ranging from chemistry to environmental science.
Intensive Properties: Intensive And Extensive Properties Worksheet
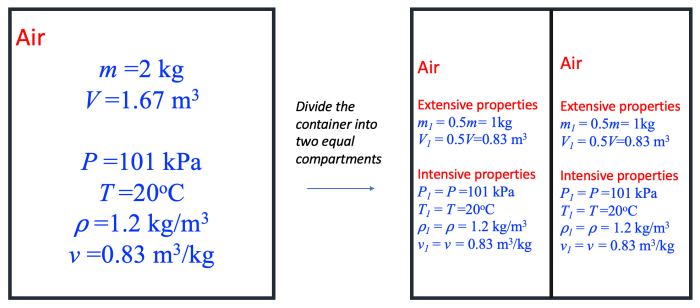
Intensive properties are physical properties that are independent of the amount of substance present. They are characteristic of the substance itself and do not change with the amount of substance present.
Examples of intensive properties include:
- Temperature
- Pressure
- Density
- Solubility
- Boiling point
Extensive Properties
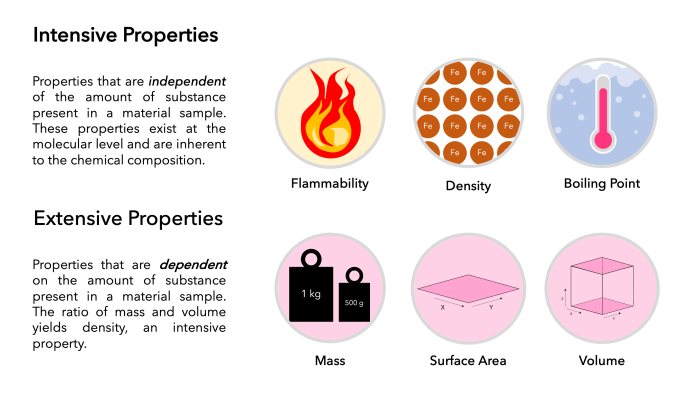
Extensive properties are physical properties that are dependent on the amount of substance present. They change with the amount of substance present.
Examples of extensive properties include:
- Mass
- Volume
- Energy
- Heat capacity
- Entropy
Comparison of Intensive and Extensive Properties

| Property Type | Definition | Examples |
|---|---|---|
| Intensive | Independent of the amount of substance present | Temperature, pressure, density |
| Extensive | Dependent on the amount of substance present | Mass, volume, energy |
Applications of Intensive and Extensive Properties

Intensive properties are used in various fields, such as chemistry and physics, to characterize substances and understand their behavior.
Extensive properties are used in various fields, such as engineering and environmental science, to design and analyze systems and processes.
Top FAQs
What is the key difference between intensive and extensive properties?
Intensive properties are independent of the amount of substance, while extensive properties are dependent on the amount of substance.
Provide an example of an intensive property.
Temperature is an example of an intensive property.
What is a practical application of extensive properties in engineering?
Extensive properties, such as mass and volume, are crucial in engineering calculations for designing structures and systems.